
Perfectly balanced mAb formulation
Achieve perfect balance in mAb formulation with tailored excipients and fluid handling systems from Avantor.® Our cGMP-grade materials ensure monoclonal antibody stability and viscosity control while minimizing risks throughout your biopharma manufacturing process. Our scalable solutions enhance manufacturing efficiency and help you optimize final formulation and fill-finish steps. With seamless supply chain security and regulatory compliance, Avantor supports your efforts to streamline production while maintaining product quality and process safety.
We can help tailor fluid handling for optimum final formulation
Avantor’s technical specialists help you optimize fluid handling in the final formulation to enhance efficiency, prevent cross-contamination during fluid transfer and minimize the risk of product loss during sampling.
Ensure a reliable supply chain for a consistent and scalable formulation
You can meet raw material supply challenges with Avantor’s reliable, high-quality excipients, buffers, and salts. We continue to expand our manufacturing capacity to better provide the critical materials you need precisely when needed. Securing your supply chain helps you maintain consistency and balance in your formulation process and ensures product quality and scalability in biopharma production.
Ensure consistent quality and minimize risk with reliable raw materials

Get a free sample of amino acid
Request a free sample of J.T.Baker® cGMP amino acids today to experience the quality that helps de-risk your supply chain. Sourced in the U.S., these high-purity amino acids provide reliable performance for biopharma applications, ensuring compliance and consistency in your production process.
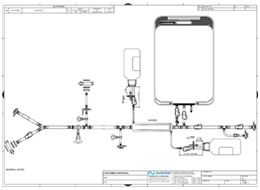
Pre-use post-sterilization integrity testing (PUPSIT)
Learn everything you should know about PUPSIT and how Avantor can guide you through the complexities of sterile filtration. Our experts can help you ensure compliance and efficiency in your biopharma processes.







