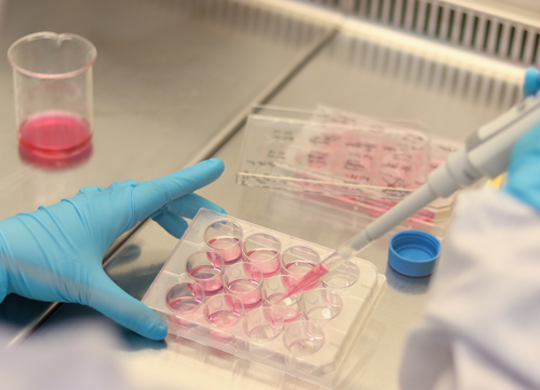
Your unique gene therapy at scale
Your scale-up process needs to be as unique as your gene therapy. We can help you customize it with the right raw materials, tools, processes, and industry experience so you can get your gene therapy from benchtop to production quickly and safely.
Avantor experts guide you on fit-for-purpose, custom solutions through every stage of development.
Pre-Clinical Development
We can help you plan your scale-up process, carefully selecting readily available and scalable cGMP materials to eliminate late-stage process changes.

J.T.Baker® Chemicals
Single-Use Technology
Process Development
Our engineering team helps you design your unique fluid path, with their in-depth knowledge of fluid handling and single-use technologiess, so you can avoid bottlenecks and create continuity as you scale.
Clinical Trials
With critical supply reliability, custom designed components and a vendor-agnostic library of materials, we can provide tailored solutions for every scenario so you can avoid production disruptions and ensure delivery to patients.

Custom Transfer Assemblies
Bioprocessing Bags
Mixing Systems

Sampling Solution
Filtration Assemblies

Innovation at Avantor
In the race to treat and cure disease, there is no room for error, and no time for delays. See our commitment to cell & gene therapy production in action.

Addressing current challenges in viral vector manufacturing
Scaling to larger volumes exacerbates manufacturing challenges, but Avantor's holistic approach helps you solve for these obstacles.

Our Quality Promise
You can depend on our proactive risk management system to deliver the highest quality of GMP raw materials and fluid handling systems. Globally aligned quality standards, regulatory support and a robust supplier audit program ensure our products are compliant, compendial and traceable, so you can scale with confidence.
Our Consultative Approach
Our experts collaborate to design your entire scale-up process tailored to your needs. With our in-depth application knowledge, we integrate discovery, development, and delivery to support an efficient and holistic gene therapy process. You can trust Avantor every step of the way.

Our Global Footprint
Avantor's global network of cGMP and ISO-certified manufacturing sites provides a powerful channel and delivery system for critical raw materials and qualified fluid handling technologies. Transparent supply chain redundancy, as well as vertically integrated products and services, ensures scale-up success.
It is essential that the raw materials used to manufacture gene therapies meet current good manufacturing practice (cGMP) quality standards and specifications to ensure productivity and patient safety. This includes quality control testing of incoming raw materials, increased documentation to show manufacturing control and robust process validation.
With new diseases emerging at record rates, it is critical to be ready and able to respond As cell and gene therapies continue to develop and get approved as treatments, standardized, safe workflows for viral vector production are critical.
The stakes are high in gene therapy, as reducing the risk of a batch failure affects patient survival directly. Leveraging Avantor’s expertise in holistic fluid handling system design, this CDMO minimized high-pressure risks in the fluid path while simplifying their overall process controls in AAV manufacturing.