Fluid-handling solutions from Avantor®
Combining innovation with efficiency to transform biologics production
Bring novel therapies to market sooner working with Avantor
Biomanufacturers need a flexible and scalable approach to fluid handling to overcome complex regulatory demands and improve production efficiency. Fluid handling solutions from Avantor, including agile single-use solutions and Masterflex® peristaltic pumps, enable biomanufacturers to bring breakthrough therapies to market quickly and safely.
Our innovative, high quality fluid handling systems are driven by the needs, challenges and aspirations of our customers. Avantor experts collaborate to design customized solutions that meet your exact process and compliance requirements, utilizing a global supply network for security and convenience.
Avantor is the only open-architecture, fluid handling provider to offer complete design, manufacturing, quality testing and logistics through every stage of product development and the biomanufacturing process. We focus on creating choice for our customers and removing the barriers of rigid, one-size-fits-all fluid handling design so you can bring critical therapies to market more quickly.
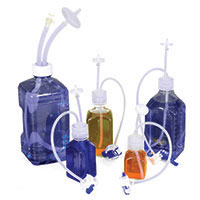
Our comprehensive product portfolio, including quality single-use systems, includes:
- Pumps
- Tubing
- Fluid path consumables
- Fluid transfer assemblies
- Specialized assemblies
- Bags and systems

Fluid handling solutions
View our complete Masterflex® product offering now
Learn more about Avantor’s complete fluid handling solutions.
Optimize accuracy and repeatability with world-class Masterflex® peristaltic pumps and fluid handling solutions.
See how our global single-use expertise, capabilities and supply chain serve every stage of biomanufacturing.
Manage the quality and risk mitigation of your biomanufacturing workflows with Avantor® Masterflex® pumps and single-use solutions.
Move your innovations forward faster with flexible, scalable solutions featuring Avantor® Masterflex® peristaltic pumps and single-use technologies.
Bring your biologics to market leveraging Avantor’s global biomanufacturing footprint to expertly manage upstream, downstream and fill/finish processes.
Our Experts Drive Quality Solutions
Our extensive expertise in fluid handling technologies means we offer cost-effective solutions, products and systems that also meet rigorous quality standards and help reduce contamination risk. There is no need to sacrifice one for the other.
Working with Avantor, you gain a strategic partner with decades of experience in fluid management pathways and regulatory compliance. We leverage that knowledge to provide comprehensive quality systems in ISO-certified, cGMP manufacturing facilities around the globe. These best practices, across all biomanufacturing workflows, ensure that you consistently receive high quality products you can trust.
Your Process, Your Way
Avantor is the leading provider of modular, highly-customized and fit-for-purpose solutions that streamline workflows at any scale and keep your production lines running. The foundation of this approach is an open architecture model, so you can mix and match a wide selection of fluid handling products from our brands or other vendors.
This comprehensive portfolio of validated, quality products, coupled with our experience across all biopharma platforms, provides the integrated, optimized systems our customers have come to rely on. Avantor’s innovation in single-use solutions, including customized solutions, and now the Masterflex suite of products, allows you to quickly develop and produce novel therapeutic treatments — from monoclonal antibodies (mAbs) to cell and gene therapies.
From direct dispense bags to peristaltic pumps, our extensive portfolio has what you need to scale from lab to production.
Global End-To-End Support
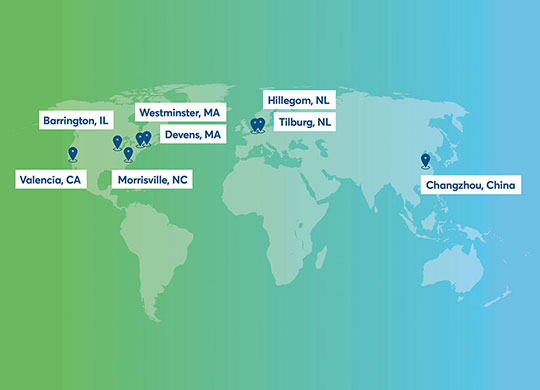
From discovery to delivery, Avantor can help you solve fluid handling challenges across the most complex bioprocessing workflow applications.
Our global capabilities and supply chain and quality programs are designed to give you peace of mind, so you can focus your time on producing critical therapies quickly and safely.
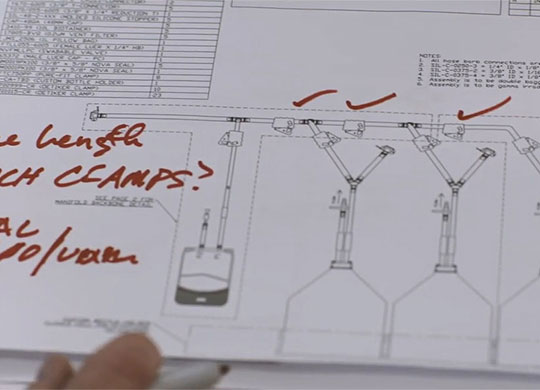
Our design and engineering experts work with your team locally to create the right system design, and then utilize our global manufacturing network to meet your needs efficiently and effectively. At every stage of the production process — upstream, downstream and fill/finish — we offer personalized support, so you have the right products on hand, right when you need them.
Avantor offers innovative fluid handling solutions with…

Quality & Choice
- Open architecture model vertically integrated on pumps, bags, tubing, stoppers and fittings supporting choice of your systems
- Comprehensive Supplier Management Program with proprietary products as well as qualified first and second sources of key components
- Complete sterility validation program to mitigate risk

Expertise
- Extensive fluid handling connectivity knowledge
- Single-use facilities
- Hybrid facilities
- Conversion from self-assembled parts
- Collaborative approach to designing your solution
Convenience
- Local single-use experts
- Expedited design and approval process
- Designs < 5 days
- Validation Packs < 5 days
- Global logistics footprint
- 100+ standard products

Customization
- Fully custom solutions designed for specific applications
- Ability to develop custom components and parts
- Customized skid systems with disposable fluid paths